Tools of the Radiocarbon Unit
AixMICADAS Spectrometer
The MICADAS (Mini Carbon Dating System) spectrometer is an innovation in the field of accelerator mass spectroscopy due to its reduced accelerator voltage of 200 kilovolts. This considerably reduces the size of the instrument, which has a footprint of 2.6 x 3.2 m². AixMICADAS is equipped with a hybrid ion source that allows the analysis of both conventional sample sizes (≈1 mgC) with graphite targets and CO2 of very small size (< 100 μgC) using the CO2 gas (GIS).
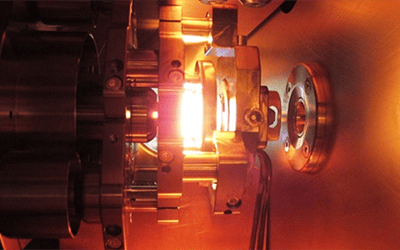
The AixMICADAS ion source is a so-called sputtering source because it uses a caesium beam to sputter the graphite pellet contained in the targets. A caesium tank is heated (T > 130 °C) to produce the caesium vapour which is then guided to a spherical ioniser heated to a very high temperature (> 1000 °C). The ioniser allows the ionisation and focusing of the caesium beam on the target. When injecting small samples in the form of CO2Special targets are used. These are drilled laterally to allow the CO2 to the sputtering site inside the target where a titanium insert allows the negative carbon ion beam to be produced. The ion source allows the insertion of 39 samples and the typical beam intensity at the source exit is 60-70 uA for graphite targets and 10-15 uA for gas targets.
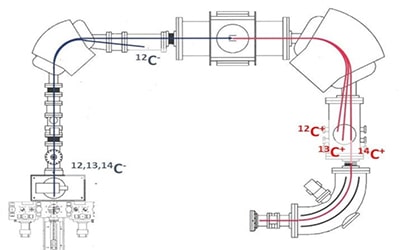
At the ion source outlet, the three carbon isotopes are injected sequentially (12C: 30 μs, 13C: 520 μs, 14C: 40 ms) in the accelerator part of the instrument thanks to a magnetic sector coupled to an ultra-fast electrostatic pulsation system. A Faraday collector at the exit of the magnet allows a measurement of the beam intensity in the low energy part of the system.
The negative ions are then directed into the accelerator part of the spectrometer where a gaseous target located in the centre of the section allows their charge state to be changed from negative to positive in order to obtain a beam accelerated to 400 keV and devoid of the vast majority of the molecular interferences of 14C (12CH2, 13CH). The use of helium as a stripping gas in the accelerator section allows for excellent beam transmission of nearly 50%. The reduced accelerator voltage of the instrument (194 kV) allows the accelerator chamber to be operated in a vacuum, rather than in SF6 insulating gas like higher energy accelerators. This greatly simplifies maintenance.
After the high-energy magnetic sector, Faraday collectors are placed in the path of the two stable carbon isotopes, 12C and 13C, in order to measure the ionic currents that will be used for radiocarbon age calculations. A special feature of MICADAS is a third Faraday collector, intermediate to the two previous ones, which measures the 13C from the molecular fragmentation of 13CH isobars of 14C. This measurement is used to control and fully correct for residual isobaric interference.
A 90° cylindrical electrostatic deflector allows a final energy selection of the beam before the AixMICADAS detector at the end of the line, which directly counts the 14C+ ions in the sample. The AixMICADAS detector is an ionisation chamber filled with isobutane gas at a pressure of 25 mbar. The ionisation chamber is separated from the accelerator vacuum by a 50 nm thick silicon nitride window. The energy of the 14C+ ions is completely dissipated in the gas volume of the detector.
CO2 gas ion source coupled to AixMICADAS
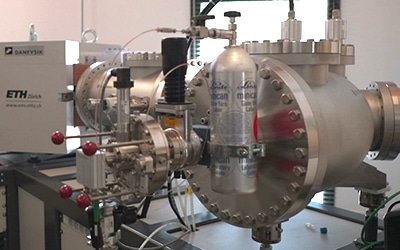
Small radiocarbon samples (< 100 µg C) are analysed as CO2 gas with the carbon dioxide source. This system for introducing CO2 (Gas Interface system, GIS, ETH Zürich) is coupled with an elemental analyser (EA), to a carbonate hydrolysis system (CHS) and to a cracker of tubes allowing different types of samples to be injected. The capture of CO2 from CHS or EA is achieved by means of a zeolite trap. After heating to 450°C, this trap releases the CO2 of the sample into an injection syringe. The CO2 is then diluted in a ratio of 95 % helium and 5 % CO2 then the mixture is injected progressively with a stepper motor into the ion source via a capillary metal tube.
A cracker of tubes allows the measurement of 14C of samples trapped as CO2 purified and sealed in glass ampoules. The cracker has 8 metal cylinders in which ampoules of 5 cm to 8 cm in length and 4 mm in diameter are inserted. A pneumatic system breaks the ampoule to release the CO2 to the injection syringe of the GIS where the CO2-He which is then injected into the AixMICADAS ion source.
An elementary analyzer (Vario Micro Cube Elementar Ó) coupled to the gas interface (GIS) allows the combustion of micro-samples (< 150 mgC) of organic matter, previously wrapped in small silver pods. After synthesis and chromatographic quantification, the CO2 product is trapped on the GIS zeolites, released by heating to 450°C, introduced and mixed with helium in the injection syringe connected to the CO2 gas from AixMICADAS.
The CHS1 carbonate hydrolysis system (Carbonate Handling System) is a device that allows the acidification (sequential or complete partial hydrolysis) of 42 samples in thermostatically controlled pillboxes (40°C). The sampling of CO2 produced by the hydrolysis is carried out using a double inlet needle that sweeps the pillbox with a helium flow (70 ml/min) and transfers the hydrolysis gas mixture to a water trap and then to the GIS zeolite trap that collects the CO2 before it is injected into the ion source.




AGE3 graphitisation system
The radiocarbon unit is equipped with a graphitisation system (AGE3) to prepare graphite targets for analysis with the AixMICADAS solid source. This system is coupled to an elemental analyzer (Vario Micro Cube Elementar Ó) and to the carbonate hydrolysis system (CHS2) to produce graphite samples from organic or inorganic materials. The CO2 from both systems is trapped on zeolites and then injected into one of the reactors by heating the trap to 420°C. Graphite is then produced at 580°C by reduction of CO2 by hydrogen in the presence of iron as a catalyst for the Bosch reaction: CO2 + 2 H2 → C + 2 H2O
To achieve a complete reaction, the water produced by the reaction is condensed in a cold finger system cooled by the Peltier effect. The automated graphitisation system allows the simultaneous reduction of 7 samples in 3 hours.
- Elemental analyzer
An elemental analyser (Vario Micro Cube Elementar Ó) allows the combustion of organic material samples (collagen, wood, soils, aerosols...) previously wrapped in small aluminium or tin pods. The sample is burnt and oxidised in a combustion column heated to 950°C under a flow of Helium. The combustion gases (SOx, NOx, H2O, O2 and CO2) are transferred by a helium stream through a reduction column filled with copper wire heated to 550ºC, thereby reducing the nitrogen oxides to N2 while sulphur and halogens are removed by silver wool. The water is then trapped in a phosphorus pentoxide tube and the residual N2 and CO2 are separated by a chromatographic column coupled to a thermal conductivity detector (TCD). The nitrogen is eluted first and then a valve system transfers the CO2 to the zeolite trap of the graphitisation system.
- Automated carbonate hydrolysis CHS2
The CHS2 is an evolution of the CHS1 system that takes the automation of carbonate analysis processes a step further. The device has 64 positions for 4.5ml pill boxes. It allows full automation of flush, leaching, hydrolysis and sample measurement procedures. The CHS2 can switch autonomously between two different acids (leaching or total hydrolysis) or syringes (flush or CO2) according to the sequence programmed by the user. The CHS2 can be used for both carbonate microsample analysis when coupled to the AixMICADAS GIS or graphite target production when coupled to the AGE3 graphitisation system.
- Pneumatic press for graphite targets
The graphite-iron mixture is then pressed into an aluminium cylinder using a pneumatic press, which produces an optimal surface for the Cs+ in the AixMICADAS ion source.

Cryogenic combustion, purification and graphitisation line
This cryogenic line, a prototype made of stainless steel, allows the preparation of small samples of CO2 (<100 µgC) prior to measurements on the AixMICADAS gas source cracker. Organic samples such as molecular fractions separated by chromatography are placed in a quartz tube in the presence of CuO (oxygen source for combustion) and silver wool to remove elements such as sulphur and halogenated compounds. The tube is then evacuated onto the vacuum line to remove CO2 The tube is transferred to a muffle furnace at about 850°C for a few hours to cause oxidation of the organic material by the CuO. The tube is transferred to a muffle furnace at about 850°C for a few hours to cause oxidation of the organic material by the CuO. The tube is then placed back on the previously evacuated line and broken in a manual tube cracker. The water produced during combustion is removed in a cryogenic trap (about -50°C) and the CO2 is trapped on a second trap (liquid nitrogen) while the non-condensable gases are vented. The CO2 previously quantified in a calibrated volume is then transferred and sealed in a glass tube suitable for the gas source cracker. This cryogenic line has also been optimised to prepare graphite targets from organic samples of standard size (≈ 1 mgC). This versatile line can be modified and extended for other applications.
EC-OC fraction separation line
This prototype consists of an analyser (Sunset Laboratory Inc) that allows the separation and quantification of organic and elemental carbon fractions from fine particles collected on atmospheric filters or other samples. The analyser is coupled to a cryogenic line for the separation and trapping of the different CO2 which are then sealed into ampoules for the measurement of 14C through the tube cracker of the CO2 gas from AixMICADAS.

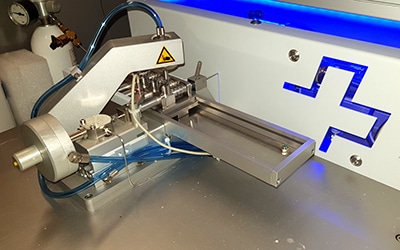
Sampling microscope
The MicroMill (Elemental Scientific) is a microscope coupled to a computer-controlled micro-drill. The MicroMill allows the sampling of carbonate archives such as corals, coralline algae, molluscs or speleothems at very high resolution (a few tens of µm).

Sample pre-treatment laboratory
Careful sampling and sample pre-treatment are crucial steps in the sample preparation process prior to radiocarbon measurements. Indeed, it is essential to completely eliminate exogenous carbon (carbon of a different age from the sample to be dated) in order not to compromise the accuracy of the measurement.
Pre-treatment methods are adapted according to the nature of the samples and contaminants, but generally the samples undergo physical treatment using, for example, a scalpel or portable milling machine (Dremel) to remove visible contaminants or outer layers of the objects to be dated, and then the samples are reduced in size (crushing, grinding or cutting) in order to increase the contact surface before the chemical pre-treatment step. The samples are pre-weighed using a balance or microbalance to determine the extraction yields.
Common chemical pre-treatment methods involve hydrolysis of carbonate samples to lose approximately 30-50 % of the initial mass. This surface cleaning aims to remove the carbonaceous fraction (from diagenetic processes such as recrystallisation of aragonite to calcite or secondary carbonate deposits) to leave only the primary carbonate for dating. Organic samples (e.g. wood and charcoal) are subjected to sequential acid-base-acid treatments to purify the original carbon. Organic samples are then freeze-dried or oven-dried, weighed and graphitised or analysed on-line from the carbon dioxide source.